Consider the following three resonance forms for the cyanate ion, (OCN) 1 - . The formal charges for each atom in the formula are labeled. 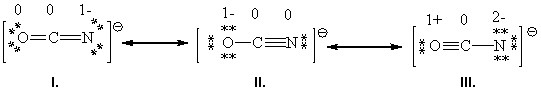 Based upon a formal charge analysis of each atom in the three resonance forms for the cyanate ion, OCN 1 - , rank the following three resonance forms with respect to increasing importance. (Least important to most important)
Based upon a formal charge analysis of each atom in the three resonance forms for the cyanate ion, OCN 1 - , rank the following three resonance forms with respect to increasing importance. (Least important to most important)
A) I < II < III
B) I < III < II
C) II < I < III
D) II < III < I
E) III < I < II
Correct Answer:
Verified
Q83: What are the formal charge and the
Q84: Given the table of electronegativity values below,
Q85: Below are two resonance forms of nitrogen
Q86: Which of the following bonds would be
Q87: Which of the following is a valid
Q89: A possible Lewis structure for BF 3
Q90: Given the three Lewis structures below, pick
Q91: What is the formal charge of the
Q92: Three possibly correct resonance forms of BrO4
Q93: Three possible resonance forms for NO₂ NH
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents