Three possible resonance forms for NO₂ NH - are shown below. Pick the best answer.
I. 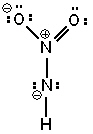
II. 
III. 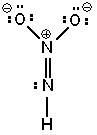
A) I and II are correct resonance forms, III is not and no others exist
B) I and II are not correct resonance forms, III is the only possible Lewis structure for this molecule
C) None of the Lewis structures shown are correct; one or more other Lewis structure(s) can be written.
D) All of the Lewis structures are correct resonance forms and no others exist.
E) All of the Lewis structures are correct resonance forms but one or more others can also be written.
Correct Answer:
Verified
Q88: Consider the following three resonance forms for
Q89: A possible Lewis structure for BF 3
Q90: Given the three Lewis structures below, pick
Q91: What is the formal charge of the
Q92: Three possibly correct resonance forms of BrO4
Q94: Three possibly correct resonance forms of N₂O4
Q95: A Lewis structure for OF 2 ,
Q96: Based upon a formal charge analysis, what
Q97: Pick the correct Lewis structure shown below.
A)
Q98: The difference in electronegativity between a boron
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents