For the reaction 2 SO₂ (g) + O₂(g) 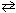 2 SO3(g) , K c = 0.15 at 300 K. What is the value of K p for this reaction?
2 SO3(g) , K c = 0.15 at 300 K. What is the value of K p for this reaction?
(R = 0.0821 L atm/(mol K)
A) 0.15
B) 6.1×10 - 3
C) 1.6×102
D) 6.7
E) 3.7
Correct Answer:
Verified
Q21: For the reaction 2 H₂ O (g)
Q22: One mole of SO₂ , two moles
Q23: A scientist places 0.0050 mol of H₂
Q24: What is the partial pressure equilibrium constant,
Q25: The value of K p is 2.72
Q27: At 525 C, the equilibrium partial pressures
Q28: An experiment begins when a researcher places
Q29: A researcher places 0.10 mol of CO
Q30: Consider the following system at equilibrium and
Q31: If K c for N₂O4 (g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents