What is the partial pressure equilibrium constant, K p , for the following reaction if the concentration equilibrium constant K c = 7.17*1015 at 200 K?
N₂(g) + 3 H₂ (g) 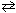 2 NH₃(g)
2 NH₃(g)
A) K p = 7.21×10 - 35
B) K p = 2.66×1013
C) K p = 4.37×1014
D) K p = 1.18×1017
E) K p = 1.93×1018
Correct Answer:
Verified
Q19: Given:
N₂(g) + 3 H₂ (g) 
Q20: The equilibrium constant for the reaction below
Q21: For the reaction 2 H₂ O (g)
Q22: One mole of SO₂ , two moles
Q23: A scientist places 0.0050 mol of H₂
Q25: The value of K p is 2.72
Q26: For the reaction 2 SO₂ (g) +
Q27: At 525 C, the equilibrium partial pressures
Q28: An experiment begins when a researcher places
Q29: A researcher places 0.10 mol of CO
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents