At 525 C, the equilibrium partial pressures for the substances in the reaction 2 SO₂ (g) + O₂(g) 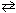 2 SO3(g) were:
2 SO3(g) were:
P SO = 0.0150 atm, P SO = 0.253 atm, and P O = 0.365 atm. The value of K p for this temperature is:
A) 1.28×10 - 3
B) 2.16×10 - 2
C) 46.2
D) 9.63×10 - 3
E) 779
Correct Answer:
Verified
Q22: One mole of SO₂ , two moles
Q23: A scientist places 0.0050 mol of H₂
Q24: What is the partial pressure equilibrium constant,
Q25: The value of K p is 2.72
Q26: For the reaction 2 SO₂ (g) +
Q28: An experiment begins when a researcher places
Q29: A researcher places 0.10 mol of CO
Q30: Consider the following system at equilibrium and
Q31: If K c for N₂O4 (g)
Q32: Consider the following reaction at 1200 K:
CH4
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents