The system is in equilibrium with the concentrations as shown at 222 C:
2 NH₃(g) 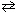 N₂(g) + 3 H₂ (g) Equilibrium pressure 0.4 atm 0.2 atm 0.6 atm By means of a cold trap, ammonia is removed until its equilibrium partial pressure is 0.1 atm. As this change occurs:
N₂(g) + 3 H₂ (g) Equilibrium pressure 0.4 atm 0.2 atm 0.6 atm By means of a cold trap, ammonia is removed until its equilibrium partial pressure is 0.1 atm. As this change occurs:
A) the position of equilibrium will shift to the right.
B) the value of the equilibrium constant will increase.
C) both answers a and b are correct.
D) the position of equilibrium will not be affected.
E) the partial pressure of hydrogen will decrease.
Correct Answer:
Verified
Q40: A researcher begins an experiment by adding
Q41: The reaction of chlorine and water vapor
Q42: Consider the reaction:
PCl 3 (g) + Cl₂
Q43: Given:
N₂(g) + 3 H₂ (g) 
Q44: Consider the following reaction at 730 K:
H₂
Q46: Consider the following chemical reaction at equilibrium:
2
Q47: For the reaction I2 (g) + Cl₂
Q48: For the reaction
2 NO (g) + 2
Q49: At 1000 K, the value of the
Q50: Under what conditions does changing the pressure
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents