The reaction of chlorine and water vapor was allowed to reach equilibrium at 25 C:
2 Cl₂ (g) + 2 H₂ O (g) 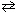 4 HCl (g) + O₂(g)
4 HCl (g) + O₂(g)
The volume of the reactor increased so that the pressure in the reaction chamber at equilibrium decreased from 5 atm to 1 atm. Choose the correct statement below.
A) The number of moles of Cl2 increase.
B) The number of moles of HCl decrease.
C) The number of moles of H2O increase.
D) The number of moles of O2 increase.
E) The number of moles of all substances remain unchanged.
Correct Answer:
Verified
Q36: For the reaction I2 (g) + Cl₂
Q37: Two (2.00) moles of NO, one (1.00)
Q38: At a certain temperature K c =
Q39: For the reaction 4 NH₃(g) + 7
Q40: A researcher begins an experiment by adding
Q42: Consider the reaction:
PCl 3 (g) + Cl₂
Q43: Given:
N₂(g) + 3 H₂ (g) 
Q44: Consider the following reaction at 730 K:
H₂
Q45: The system is in equilibrium with the
Q46: Consider the following chemical reaction at equilibrium:
2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents