Given:
N₂(g) + 3 H₂ (g) 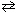 2 NH₃(g) K p (450 C) = 4.51*10- 5 At a single moment, if the partial pressure values for these three components were:
2 NH₃(g) K p (450 C) = 4.51*10- 5 At a single moment, if the partial pressure values for these three components were:
PH 2= 129 atm P N2 = 38 atm P NH3 = 171 atm What can be stated about the reaction quotient , Q , and the direction that the reaction is preceding?
A) Q = 3.49×10 - 2 and the reaction is preceding from left to right as written.
B) Q = 3.49×10 - 2 and the reaction is preceding from right to left as written.
C) Q = 3.58×10 - 4 and the reaction is preceding from left to right as written.
D) Q = 3.58×10 - 4 and the reaction is preceding from right to left as written.
E) Q = 4.51×10 - 5 and the reaction is at equilibrium.
Correct Answer:
Verified
Q38: At a certain temperature K c =
Q39: For the reaction 4 NH₃(g) + 7
Q40: A researcher begins an experiment by adding
Q41: The reaction of chlorine and water vapor
Q42: Consider the reaction:
PCl 3 (g) + Cl₂
Q44: Consider the following reaction at 730 K:
H₂
Q45: The system is in equilibrium with the
Q46: Consider the following chemical reaction at equilibrium:
2
Q47: For the reaction I2 (g) + Cl₂
Q48: For the reaction
2 NO (g) + 2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents