Which reaction shown below is shifted in the direction of the products (to the right) by increasing the pressure of the reaction mixture?
A) H₂ (g) + C₂ N₂(g) 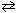 2 HCN (g)
2 HCN (g)
B) CO (g) + Br₂(g) 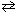 CO Br₂(g)
CO Br₂(g)
C) CH4 (g) + 2 H₂ S (g) 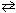 C S2(g) + 4 H₂ (g)
C S2(g) + 4 H₂ (g)
D) 2 Na2 O (s) 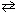 4 Na (
4 Na ( 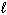 ) + O₂(g)
) + O₂(g)
E) None of these.
Correct Answer:
Verified
Q75: Which of the following reactions is product
Q76: At constant volume, helium gas was injected
Q77: For the reaction H₂ (g) + I2
Q78: At equilibrium, the endothermic reaction N₂(g) +
Q79: At 600 C, K p = 0.57
Q81: A reaction vessel is charged witH₂ .46
Q82: At high temperature, the following gas phase
Q83: Enough NO gas was placed into a
Q84: What is the equilibrium partial pressure of
Q85: One mole of HI is placed in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents