A reaction vessel is charged witH₂ .46 atm of NO, 2.46 atm of H₂ O and 1.23 atm of H₂ gases. Equilibrium is established as shown in the reaction below. 2 NO (g) + 2 H₂ (g) 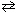 N₂(g) + 2 H₂ O (g)
N₂(g) + 2 H₂ O (g)
At equilibrium, the partial pressure of NO is 1.53 atm . What is the value of the equilibrium constant, K p ?
A) 3.94×10 - 2
B) 3.43
C) 25.4
D) 28.6
E) 50.8
Correct Answer:
Verified
Q76: At constant volume, helium gas was injected
Q77: For the reaction H₂ (g) + I2
Q78: At equilibrium, the endothermic reaction N₂(g) +
Q79: At 600 C, K p = 0.57
Q80: Which reaction shown below is shifted in
Q82: At high temperature, the following gas phase
Q83: Enough NO gas was placed into a
Q84: What is the equilibrium partial pressure of
Q85: One mole of HI is placed in
Q86: Some N₂H4 is placed in a 1.0
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents