Enough NO gas was placed into a 1.00 liter container such that the pressure was 0.750 atm, and the following reaction was allowed to reach equilibrium:
2 NO (g) 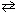 N₂(g) + O₂(g)
N₂(g) + O₂(g)
If the equilibrium pressure of O₂was 5.79 10 - 2 atm, what is the value of K p for this reaction?
A) 5.96×10 - 3
B) 11.0
C) 9.13×10 - 2
D) 8.33×10 - 3
E) 1.20×102
Correct Answer:
Verified
Q78: At equilibrium, the endothermic reaction N₂(g) +
Q79: At 600 C, K p = 0.57
Q80: Which reaction shown below is shifted in
Q81: A reaction vessel is charged witH₂ .46
Q82: At high temperature, the following gas phase
Q84: What is the equilibrium partial pressure of
Q85: One mole of HI is placed in
Q86: Some N₂H4 is placed in a 1.0
Q87: Consider the following reaction and its corresponding
Q88: At 25 C, K p = 0.240
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents