For the reaction 2 KClO3(s) 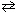 2 KCl (s) + 3 O₂(g) , K c = 2.0 at 750 C. What is the equilibrium concentration of O₂?
2 KCl (s) + 3 O₂(g) , K c = 2.0 at 750 C. What is the equilibrium concentration of O₂?
A) 2.0 M
B) (2.0) 1/3 M
C) (2.0) - 1/3 M
D) 8.0 M
E) (8.0) - 1 M
Correct Answer:
Verified
Q155: For the equilibrium reaction
AgCl (s) 
Q156: What is the solubility product expression for
Q157: An endothermic reaction CaCO3(s) Q158: In water saturated with BaF2 at a Q159: At a certain temperature, for the reaction Q161: At a particular temperature, the K sp Q162: The solubility of calcium arsenate, Ca3(AsO4)2, in Q163: What is the solubility product constant, K Q164: The molar solubility of SrF2 in water Q165: The solubility product constant for Ca3(PO4)2 is![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents