Passage Ribulose-1,5-Bisphosphate Carboxylase/oxygenase (Rubisco) Is One of the Most Abundant Enzymes
Passage
Ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) is one of the most abundant enzymes on Earth. In carbon-fixing organisms, it consumes CO2 to carboxylate the sugar ribulose-1,5-bisphosphate (Ru1,5BP) , and forms two molecules of 3-phosphoglycerate (3PG) as a product. The functional form of rubisco from the bacterium Rhodospirillum rubrum is a noncovalent homodimer composed of two 51 kDa monomers. Each subunit requires a magnesium ion as a prosthetic group in the active site.Rubisco is often used as a model for protein folding studies. On their own, rubisco monomers typically cannot fold to completion and instead are trapped in a form known as a "kinetic intermediate." These kinetically trapped monomers cannot dimerize correctly and are prone to aggregation.Scientists interested in studying protein folding dynamics labeled the rubisco kinetic intermediate with a set of fluorophores known as a FRET (fluorescence resonance energy transfer) pair, with one fluorophore at the N-terminus and the other at the C-terminus. A FRET signal occurs when the emission spectrum of one fluorophore (the donor) overlaps with the excitation spectrum of the other (the acceptor) . When the two fluorophores are sufficiently close to each other, the donor can transfer energy from the light it absorbs directly to the acceptor, causing the acceptor to emit light when the donor absorbs light.The GroEL/ES complex is a chaperone protein that binds and releases misfolded proteins, hydrolyzing ATP each time the protein is released. A misfolded protein may undergo multiple rounds of binding and release before adopting its correct conformation, or it may never fold correctly and instead be targeted for destruction (Figure 1) .
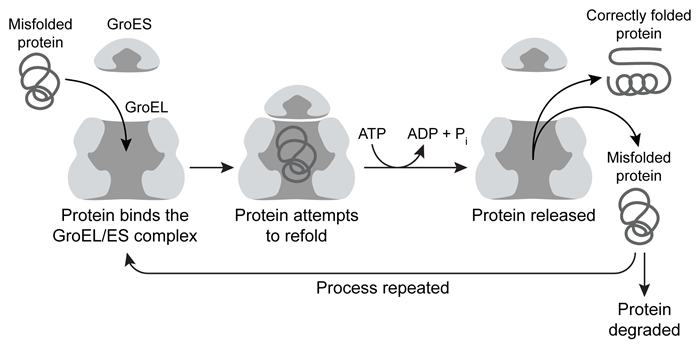 Figure 1 Representation of GroEL/ES-mediated protein foldingResearchers diluted the labeled kinetic intermediate into a refolding buffer in the presence or absence of 200 nM GroEL/ES and then monitored the FRET signal over time. The results are shown in Figure 2.
Figure 1 Representation of GroEL/ES-mediated protein foldingResearchers diluted the labeled kinetic intermediate into a refolding buffer in the presence or absence of 200 nM GroEL/ES and then monitored the FRET signal over time. The results are shown in Figure 2.
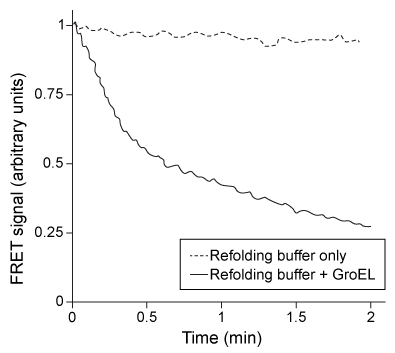 Figure 2 FRET signal of labeled rubisco with and without GroEL
Figure 2 FRET signal of labeled rubisco with and without GroEL
Lin Z, Rye HS. Expansion and compression of a protein folding intermediate by GroEL. Mol Cell. 2004;16(1) :23-34.
-Which of the following experiments, performed in a closed system, could confirm that GroEL/ES successfully facilitated proper rubisco folding?
A) Adding Ru1,5BP and measuring CO2 consumption over time
B) Running rubisco on an SDS gel to assess its dimerization
C) Observing a decrease in the concentration of inorganic phosphate
D) Measuring the Kd of GroEL/ES interactions with Mg2+ over time
Correct Answer:
Verified
Q76: Passage
Although the structure of the cell membrane
Q77: Passage
Upon entering muscle cells, glucose is immediately
Q78: Passage
Although the structure of the cell membrane
Q79: Passage
Dystroglycan (Dg) is a transmembrane protein that
Q80: Passage
Upon entering muscle cells, glucose is immediately
Q82: Passage
Myalgic encephalopathy (ME), or chronic fatigue syndrome
Q83: Passage
Synthesis of coenzyme A (CoA) requires vitamin
Q84: Passage
Ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) is one of the
Q85: If a frameshift mutation changes the number
Q86: Passage
Myalgic encephalopathy (ME), or chronic fatigue syndrome
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents