Passage
Nuclear medicine uses radiopharmaceuticals for disease treatment and as tracers in medical imaging studies. Yttrium-90 (90Y) is a radiopharmaceutical agent used to treat overgrown joint lining known as pigmented villonodular synovitis, as well as some forms of liver cancer. It has a half-life of about 64 hours.Although the relatively short half-life of 90Y is optimal for use in medical applications, transportation and storage of the radiopharmaceutical are not feasible. As a result, strontium-90 (90Sr) , with a half-life of 28.8 years, is the most common source of 90Y. 90Sr is created by a nuclear fission process that begins with uranium-235 (235U) in a nuclear reactor as shown in Reaction 1.235U → 90Sr + ZReaction 1Fission of 235U produces 90Sr as well as additional fission products (Z) including, but not limited to, technetium-99 (99Tc) , iodine-129 (129I) , and zirconium-93 (93Zr) . Figure 1 shows the distribution of fission products.
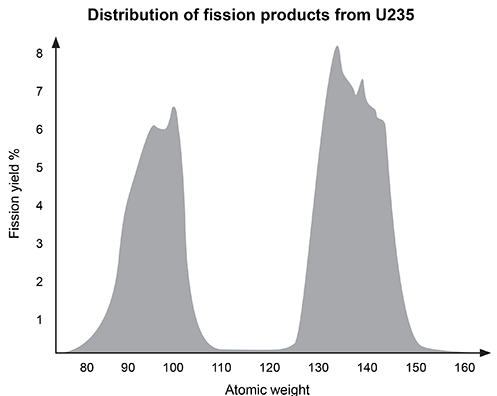 Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Adapted from Wheeler CE. Comments on vaccines, August 1987. J Am Acad Dermatol. 1988;18(1 Pt 2) :232-4.
-90Y converts to 90Zr by beta decay. Which of the following elements exhibits chemical properties most similar to zirconium-90? (Note: Assume that 90Zr gains any needed valence electrons from its environment immediately after being formed by the nuclear conversion.)
A) 90Nb
B) 174Hf
C) 46Sc
D) 48Ca
Correct Answer:
Verified
Q15: Passage
Nuclear medicine uses radiopharmaceuticals for disease treatment
Q16: Passage
Depending on the active compound's specific method
Q17: Passage
Nuclear medicine uses radiopharmaceuticals for disease treatment
Q18: Passage
The bicarbonate (HCO3−) buffer system (Reaction 1)
Q19: Passage
The bicarbonate (HCO3−) buffer system (Reaction 1)
Q21: Passage
In polluted urban environments, airborne proteins can
Q22: Passage
Umami is one of five basic tastes
Q23: Passage
Catabolism is an oxidative process in which
Q24: Passage
Catabolism is an oxidative process in which
Q25: Passage
In polluted urban environments, airborne proteins can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents