Passage
In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O3) and nitrogen dioxide (NO2) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO2 by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1.
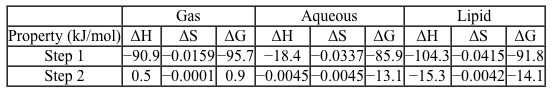
 Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
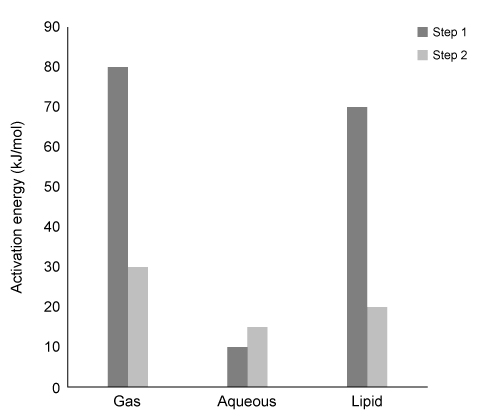 Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
 Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13) :3479-90.
Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13) :3479-90.
-Because step 2 of the reaction in aqueous phase is rate-limiting, the formation of ROI from tyrosine and ozone in step 1 can be assumed to reach a state of equilibrium.  Tyrosine(aq) + O3(aq) ⇌ ROI(aq) + HO3(aq) Which of the following will occur if the temperature of this system is raised from 298.15 K to 350 K?
Tyrosine(aq) + O3(aq) ⇌ ROI(aq) + HO3(aq) Which of the following will occur if the temperature of this system is raised from 298.15 K to 350 K?
A) Keq will increase.
B) The reaction will be driven toward the reactants.
C) The reaction will be driven toward the products.
D) Keq will remain the same.
Correct Answer:
Verified
Q20: Passage
Nuclear medicine uses radiopharmaceuticals for disease treatment
Q21: Passage
In polluted urban environments, airborne proteins can
Q22: Passage
Umami is one of five basic tastes
Q23: Passage
Catabolism is an oxidative process in which
Q24: Passage
Catabolism is an oxidative process in which
Q26: Passage
Catabolism is an oxidative process in which
Q27: Passage
In polluted urban environments, airborne proteins can
Q28: Passage
Umami is one of five basic tastes
Q29: Passage
Umami is one of five basic tastes
Q30: Passage
Depending on the active compound's specific method
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents