Passage
Depending on the active compound's specific method of action, sunscreens are broadly classified into two groups: organic and inorganic. Organic sunscreens typically contain aromatic compounds that absorb ultraviolet (UV) radiation. The absorbed energy from the UV radiation causes these compounds to enter electronically excited states which then relax back to the ground state and harmlessly re-emit the absorbed radiation as heat. Alternatively, inorganic sunscreens contain metallic nanoparticles that serve to reflect, scatter, and absorb incident radiation. The mechanism for UV protection of each group is shown in Figure 1.
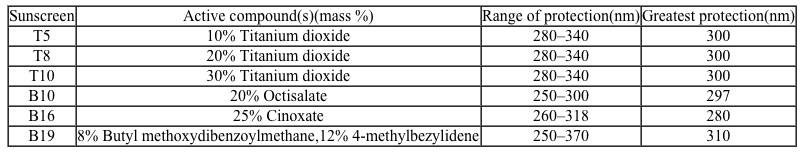
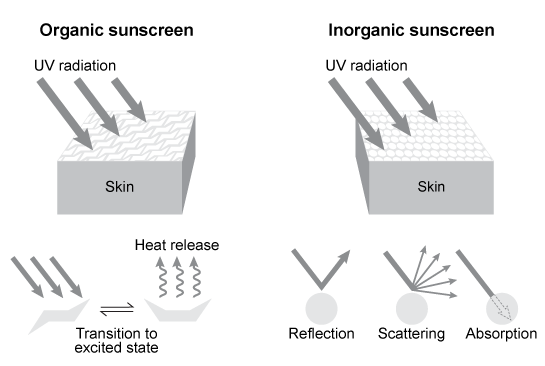 Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF) . However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF) . However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
 In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
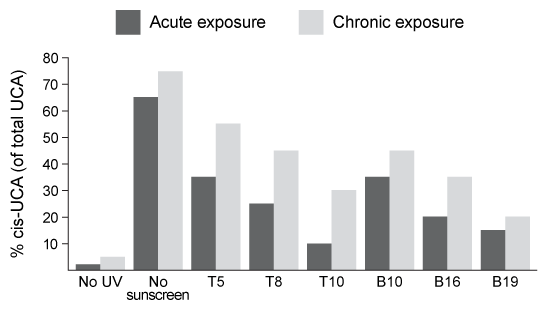 Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Adapted from Van der molen RG, Out-luiting C, Driller H, Claas FH, Koerten HK, Mommaas AM. Broad-spectrum sunscreens offer protection against urocanic acid photoisomerization by artificial ultraviolet radiation in human skin. J Invest Dermatol. 2000;115(3) :421-6.
-Which of the following is true concerning the energy difference ΔE between the ground state and the photo-excited state that occurs most often for the active compound in sunscreen B10?
A) ΔE=hc×297 nm
B) ΔE=hc×(250 nm−300 nm)
C) 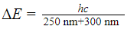
D) 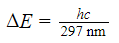
Correct Answer:
Verified
Q25: Passage
In polluted urban environments, airborne proteins can
Q26: Passage
Catabolism is an oxidative process in which
Q27: Passage
In polluted urban environments, airborne proteins can
Q28: Passage
Umami is one of five basic tastes
Q29: Passage
Umami is one of five basic tastes
Q31: Passage
Catabolism is an oxidative process in which
Q32: Passage
Depending on the active compound's specific method
Q33: Passage
Umami is one of five basic tastes
Q34: Passage
Catabolism is an oxidative process in which
Q35: Passage
In polluted urban environments, airborne proteins can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents