Passage
Catabolism is an oxidative process in which electrons are transferred from lipids, proteins, and carbohydrates to generate high-energy electron carriers from nicotinamide adenine dinucleotide (NADH/NAD+) and flavin adenine dinucleotide (FADH2/FAD) . Both are used in the electron transport chain (ETC) as electron providers in a series of reactions. The transfer of electrons provides energy that drives oxidative phosphorylation and the production of ATP. Reduction of NAD+ proceeds according to the following half-reaction:
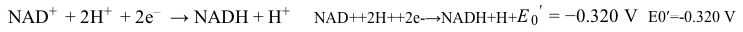 Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
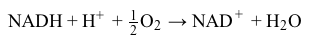 NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf) , that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf) , that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
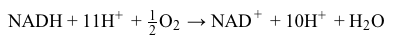 NADH+11H++12O2→NAD++10H++H2OReaction 3
NADH+11H++12O2→NAD++10H++H2OReaction 3
Adapted from Garrett R, Grisham CM, Sabat M. Biochemistry. Cengage Learning; 2011.
-For biological processes, which of the following is true of oxidation-reduction reactions?Reduction often involves the formation of bonds to hydrogen.Oxidation often involves the formation of bonds to oxygen.Reducing agents undergo a change to a lower oxidation state.
A) I only
B) I and II only
C) II and III only
D) I, II, and III
Correct Answer:
Verified
Q21: Passage
In polluted urban environments, airborne proteins can
Q22: Passage
Umami is one of five basic tastes
Q23: Passage
Catabolism is an oxidative process in which
Q24: Passage
Catabolism is an oxidative process in which
Q25: Passage
In polluted urban environments, airborne proteins can
Q27: Passage
In polluted urban environments, airborne proteins can
Q28: Passage
Umami is one of five basic tastes
Q29: Passage
Umami is one of five basic tastes
Q30: Passage
Depending on the active compound's specific method
Q31: Passage
Catabolism is an oxidative process in which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents