Passage
Water is unique in that all three of its phases exist over a small range of pressures and temperatures, as shown in Figure 1.
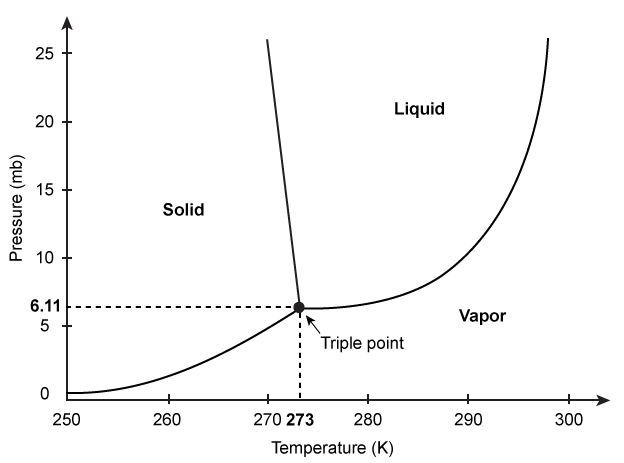 Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
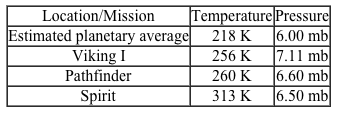 Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL) , are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure) .The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL) , are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure) .The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
-Which of the following molecules is unable to form hydrogen bonds with water?
A) C2H4Br2
B) C3H9N
C) C9H18O2
D) C2F4
Correct Answer:
Verified
Q36: Passage
Umami is one of five basic tastes
Q37: Passage
In polluted urban environments, airborne proteins can
Q38: Passage
Depending on the active compound's specific method
Q39: Passage
In polluted urban environments, airborne proteins can
Q40: Passage
Catabolism is an oxidative process in which
Q42: Passage
Heme is found in several proteins, including
Q43: Passage
Water is unique in that all three
Q44: Passage
Heme is found in several proteins, including
Q45: Passage
Bone growth and remodeling during fracture healing
Q46: Passage
Water is unique in that all three
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents