Passage
Heme is found in several proteins, including hemoglobin in blood and myoglobin in muscle. It has a four-coordinate iron-bound porphyrin structure where ferrous iron (Fe2+) is bound to four nitrogen atoms through coordinate covalent bonds. The most common heme found in the body is heme b in hemoglobin (Figure 1) .
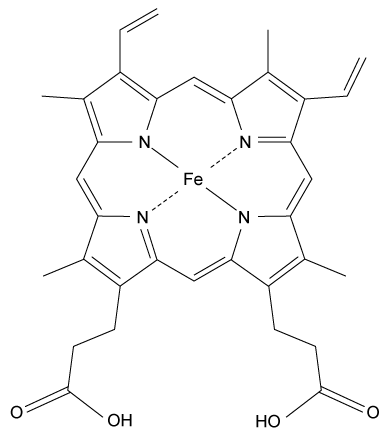 Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP) . ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP) . ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
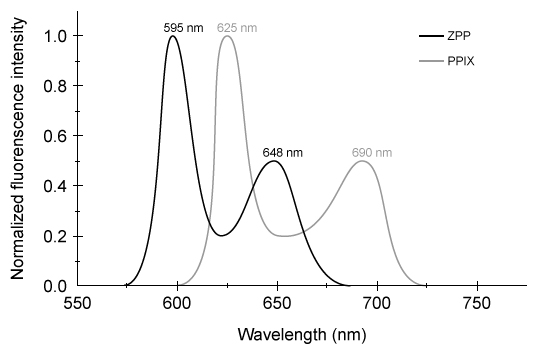 Figure 2 ZPP and PPIX fluorescence emission spectra
Figure 2 ZPP and PPIX fluorescence emission spectra
Adapted from Hennig G, Gruber C, Vogeser M, et al. Dual-wavelength excitation for fluorescence-based quantification of zinc protoporphyrin IX and protoporphyrin IX in whole blood. J Biophotonics. 2014;7(7) :514-24.
-Which of the following does NOT describe the iron-porphyrin complex formed between Fe2+ and the four nitrogen atoms in Figure 1?
A) Each nitrogen atom provides both bonding electrons in each coordinate bond.
B) The net charge of -the iron-porphyrin complex is +2.
C) Nitrogen's electrons interact with iron's d orbitals.
D) Iron's electron configuration determines coordinate bond strength.
Correct Answer:
Verified
Q37: Passage
In polluted urban environments, airborne proteins can
Q38: Passage
Depending on the active compound's specific method
Q39: Passage
In polluted urban environments, airborne proteins can
Q40: Passage
Catabolism is an oxidative process in which
Q41: Passage
Water is unique in that all three
Q43: Passage
Water is unique in that all three
Q44: Passage
Heme is found in several proteins, including
Q45: Passage
Bone growth and remodeling during fracture healing
Q46: Passage
Water is unique in that all three
Q47: Passage
Household cleaners commonly contain either ammonia (NH3)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents