Passage
Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H2O(l) → Mg(OH) 2(s) + H2(g) Reaction 1 Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction:
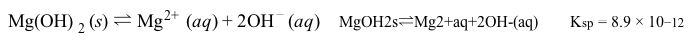 Reaction 2 Solubility equilibrium of Mg(OH) 2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
Reaction 2 Solubility equilibrium of Mg(OH) 2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
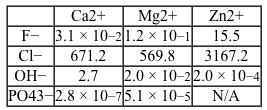 Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9) :2623-35.
Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9) :2623-35.
-Throughout the experiment, researchers monitored the amount of magnesium that dissolved at various time points. Which of the following is NOT a valid test for the rate of magnesium corrosion?
A) Periodically measure the volume of H2 gas evolved from the reaction.
B) Periodically measure the mass of the magnesium disks.
C) Periodically measure Mg2+ concentration in the SBF using elemental analysis techniques.
D) Periodically measure the amount of OH− produced by titrating the beaker contents with HCl.
Correct Answer:
Verified
Q40: Passage
Catabolism is an oxidative process in which
Q41: Passage
Water is unique in that all three
Q42: Passage
Heme is found in several proteins, including
Q43: Passage
Water is unique in that all three
Q44: Passage
Heme is found in several proteins, including
Q46: Passage
Water is unique in that all three
Q47: Passage
Household cleaners commonly contain either ammonia (NH3)
Q48: Passage
Bone growth and remodeling during fracture healing
Q49: Passage
Heme is found in several proteins, including
Q50: Passage
Bone growth and remodeling during fracture healing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents