Passage
Kidney stones are a common ailment affecting approximately 10% of adults in the United States. They form when solutes precipitate out of solution as crystals in the urinary tract, and they can cause severe pain in the side, back, abdomen, and groin. Individuals who have been previously diagnosed with kidney stones have an increased probability of developing new stones relative to unaffected individuals. Different measures may help prevent the formation of different kinds of stones, so analysis of the composition of stones that have been passed or removed can aid in preventing recurrence. Stones can be ground into fine powders, dissolved in a small amount of solvent, and analyzed by infrared (IR) spectroscopy, as shown in Figure 1.
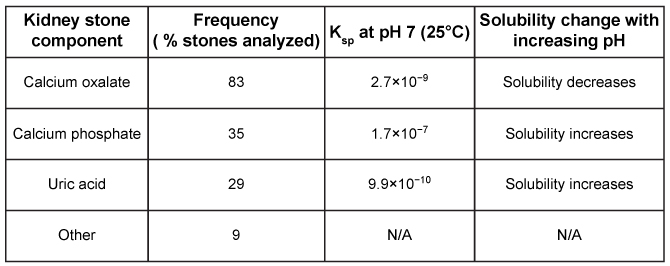
 Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters
Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters
 Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate.
Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate.
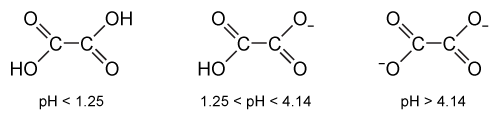 Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7) 2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.
Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7) 2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.
Adapted from Primiano A, Persichilli S, Gambaro G, et al. FT-IR analysis of urinary stones: a helpful tool for clinician comparison with the chemical spot test. Dis Markers. 2014;2014:176165.
-Given the unbalanced equation (Reaction 1) and the molecular weight of calcium citrate (498.5 g/mol) , if 15 nmol of calcium oxalate is mixed with 15 nmol of potassium citrate, what is the approximate theoretical yield of calcium citrate?
A) 1,250 ng
B) 2,500 ng
C) 3,750 ng
D) 7,500 ng
Correct Answer:
Verified
Q82: The bonds of four salts (MgBr2, NaCl,
Q83: Passage
Dmitri Mendeleev is credited with grouping the
Q84: Sebacic acid (HOOC−(CH2)8−COOH) is a naturally occurring
Q85: Ozone (O3) in the atmosphere protects against
Q86: Researchers wished to mimic the conditions of
Q88: Passage
Dmitri Mendeleev is credited with grouping the
Q89: Passage
Dmitri Mendeleev is credited with grouping the
Q90: A reaction was performed in which compounds
Q91: An 84-mg sample of a compound is
Q92: Antacids act by functioning chemically as weak
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents