What are the correct formal charges for nitrogen atoms I, II, and III, respectively, in the azide below? 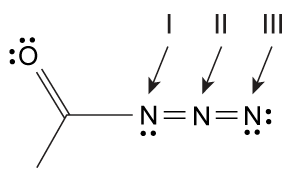
A) 0, +1, −1
B) +1, 0, −1
C) 0, −1, +1
D) −1, +1, 0
Correct Answer:
Verified
Q158: Passage
The alkali metals are a highly reactive
Q159: Passage
The alkali metals are a highly reactive
Q160: Passage
The alkali metals are a highly reactive
Q161: Passage
The primary structure of a protein is
Q162: Some test strips for blood glucose meters
Q164: If MgCO3 undergoes a double replacement reaction
Q165: A 0.040 M HCl solution is measured
Q166: Passage
The primary structure of a protein is
Q167: The electrolysis of aqueous silver nitrate involves
Q168: Oxalate anions, C2O42−, added to a solution
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents