Passage The Radiopharmaceutical 2-Deoxy-2-(18F)fluoro-D-Glucose (Abbreviated as 18F-FDG) Is a Modified Form
Passage
The radiopharmaceutical 2-deoxy-2-(18F) fluoro-D-glucose (abbreviated as 18F-FDG) is a modified form of D-glucose in which the hydroxyl group on the 2-carbon of the molecule has been replaced with a positron-emitting 18F radionuclide. Synthesis of 18F-FDG is accomplished in several steps involving both nuclear and chemical reactions, as shown in Figure 1.
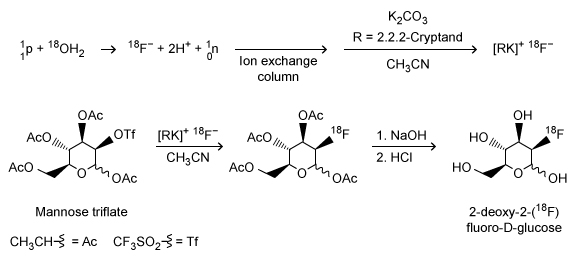 Figure 1 Synthetic scheme for 2-deoxy-2-(18F) fluoro-D-glucoseInitially, a target of 18O-enriched water (18OH2) is bombarded by protons using a cyclotron to achieve a (p-n) nuclear reaction in which a proton hitting the 18O nucleus is absorbed by the nucleus, causing the ejection of a neutron and forming an 18F nucleus. Following separation of the resulting 18F− anions from the residual 18OH2 via an ion exchange column, 18F− is used in a nucleophilic substitution reaction with an acetylated mannose triflate substrate. Subsequent hydrolysis to remove the acetyl protecting groups yields 18F-FDG.Because 18F-FDG is an analog of D-glucose, it is readily taken into cells by the same metabolic processes as D-glucose. Once inside the cells, the positron emissions of the 18F radionuclide can be detected by positron emission tomography (PET) scans to produce diagnostic medical images of objects such as tumors. Emissions from the 18F radionuclide subsequently diminish over time via radioactive decay (Figure 2) .
Figure 1 Synthetic scheme for 2-deoxy-2-(18F) fluoro-D-glucoseInitially, a target of 18O-enriched water (18OH2) is bombarded by protons using a cyclotron to achieve a (p-n) nuclear reaction in which a proton hitting the 18O nucleus is absorbed by the nucleus, causing the ejection of a neutron and forming an 18F nucleus. Following separation of the resulting 18F− anions from the residual 18OH2 via an ion exchange column, 18F− is used in a nucleophilic substitution reaction with an acetylated mannose triflate substrate. Subsequent hydrolysis to remove the acetyl protecting groups yields 18F-FDG.Because 18F-FDG is an analog of D-glucose, it is readily taken into cells by the same metabolic processes as D-glucose. Once inside the cells, the positron emissions of the 18F radionuclide can be detected by positron emission tomography (PET) scans to produce diagnostic medical images of objects such as tumors. Emissions from the 18F radionuclide subsequently diminish over time via radioactive decay (Figure 2) .
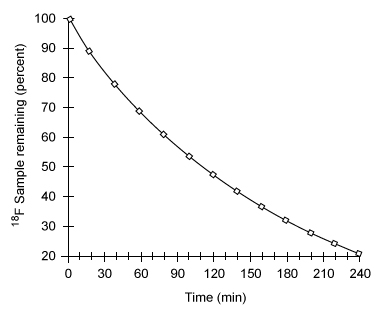 Figure 2 Nuclear decay of the 18F radionuclideBecause of limitations in the spatial resolution of PET scanners, when an object (such as a tumor) is smaller than 30 mm in diameter, the measured specific uptake value (SUV) of 18F-FDG accumulated within the object is lower than the actual SUV. The recovery coefficient (the ratio of the measured SUV to the actual SUV) shows that the measured SUV decreases as the size of the object decreases below the 30-mm threshold (Figure 3) .
Figure 2 Nuclear decay of the 18F radionuclideBecause of limitations in the spatial resolution of PET scanners, when an object (such as a tumor) is smaller than 30 mm in diameter, the measured specific uptake value (SUV) of 18F-FDG accumulated within the object is lower than the actual SUV. The recovery coefficient (the ratio of the measured SUV to the actual SUV) shows that the measured SUV decreases as the size of the object decreases below the 30-mm threshold (Figure 3) .
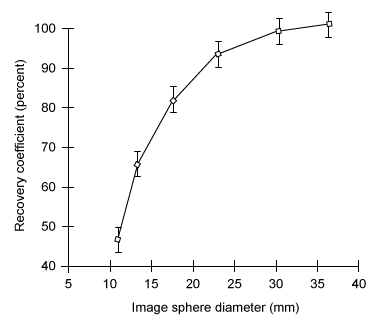 Figure 3 Recovery coefficient as a function of object size in a PET scan with a spatial resolution of 7 mm using ordered subsets expectation maximization (OSEM) image processing
Figure 3 Recovery coefficient as a function of object size in a PET scan with a spatial resolution of 7 mm using ordered subsets expectation maximization (OSEM) image processing
Adapted from: S. Yu. "Review of F-FDG Synthesis and Quality Control." Biomed Imaging Interv J. ©2006 Department of Biomedical Imaging, Faculty of Medicine, University of Malaysa; and P. E. Kinahan, et al., "Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy." Semin Ultrasound CT MR. ©2010 Elsevier.
-Which of the following products is formed from 18F-FDG following a positron emission? (Note: Assume any anions produced acquire hydrogen ions from the aqueous physiological environment.)
A) 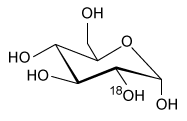
B) 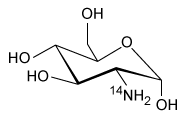
C) 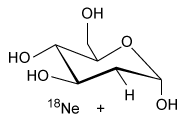
D) 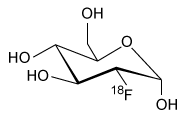
Correct Answer:
Verified
Q245: Passage
The tendency of a chemical species to
Q246: Passage
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
Q247: Passage
The tendency of a chemical species to
Q248: Which of the following compound pairs, dissolved
Q249: Passage
Living organisms that require oxygen to respire
Q251: Passage
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
Q252: The first step of a nitration reaction
Q253: Passage
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
Q254: The overall reaction for the generation of
Q255: Passage
The tendency of a chemical species to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents