Passage
The tendency of a chemical species to undergo reduction during an electrochemical reaction is indicated by the standard potential E° for the reaction. As represented generally by Reaction 1, E° measures the potential Z at which element X with oxidation number N accepts N electrons and is reduced to its elemental state.X(N) + Ne− → X(0) , E° = Z voltsReaction 1In a galvanic cell, E° > 0 indicates a spontaneous reaction. Therefore, chemical species with higher positive E° values are reduced more easily than those with lower E° values.Using E° as a basis of comparison, the relative thermodynamic stabilities of different chemical species involving the same element at different oxidation states can be presented graphically using a Frost diagram. For a series of compounds containing element X, a Frost diagram plots the value of NE° for each compound against the corresponding oxidation number N of element X within the species. Frost diagrams for several species containing manganese or chlorine are shown in Figure 1.
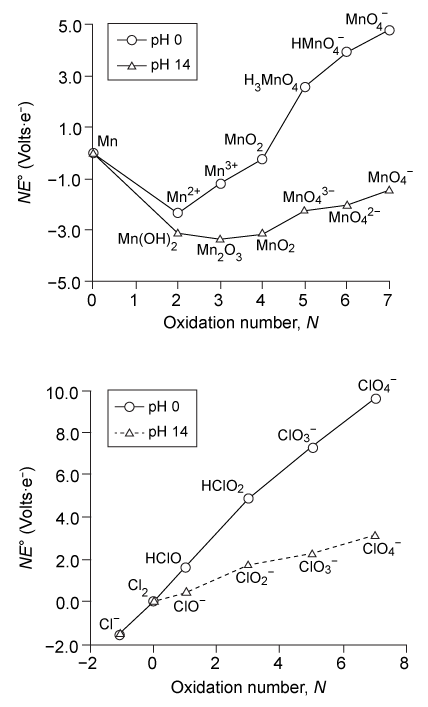 Figure 1 Frost diagrams for manganese and chlorineOn a Frost diagram, NE° is proportional to the standard Gibbs free energy ΔG° according to the relationshipΔG° = −FNE° = −nFE°Equation 1where n is the number of moles of electrons transferred during the electrochemical process and F is the Faraday constant. Free energy considerations also show that a species is prone to disproportionation if its position on the Frost diagram lies above a line connecting the points of two adjacent species, as seen for species B in Figure 2.
Figure 1 Frost diagrams for manganese and chlorineOn a Frost diagram, NE° is proportional to the standard Gibbs free energy ΔG° according to the relationshipΔG° = −FNE° = −nFE°Equation 1where n is the number of moles of electrons transferred during the electrochemical process and F is the Faraday constant. Free energy considerations also show that a species is prone to disproportionation if its position on the Frost diagram lies above a line connecting the points of two adjacent species, as seen for species B in Figure 2.
 Figure 2 General Frost diagram for an element forming species A, B, C, and D.The slope of a line segment joining two species on a Frost diagram is equal to the standard reduction potential for the couple. A greater slope indicates a higher corresponding reduction potential. As a result, in Figure 2, the reduction potential for B to A is lower than that for D to C.
Figure 2 General Frost diagram for an element forming species A, B, C, and D.The slope of a line segment joining two species on a Frost diagram is equal to the standard reduction potential for the couple. A greater slope indicates a higher corresponding reduction potential. As a result, in Figure 2, the reduction potential for B to A is lower than that for D to C.
-3 H2O + 5 ClO3− + 3 I2 → 5 Cl− + 6 IO3− + 6 H+Consider the reaction of iodine with ClO3− under acidic conditions, as shown above. If 6 moles of electrons are required to reduce 1 mole of ClO3−, how many moles of electrons are required to completely reduce all the ClO3− ions present in 250 mL of a 4.8 M ClO3− solution?
A) 7.2 moles
B) 6.0 moles
C) 4.8 moles
D) 1.2 moles
Correct Answer:
Verified
Q250: Passage
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
Q251: Passage
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
Q252: The first step of a nitration reaction
Q253: Passage
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
Q254: The overall reaction for the generation of
Q256: Which of the following pressure measurements is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents