The overall reaction for the generation of an NO2+ electrophile: 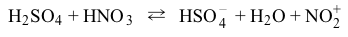 can be stated in two separate, reversible steps as a protonation followed by a decomposition (Reactions 1 and 2) .
can be stated in two separate, reversible steps as a protonation followed by a decomposition (Reactions 1 and 2) . Reaction 1
Reaction 1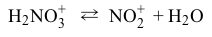 Reaction 2Which molecule acts as a Lewis acid, but NOT as a Brønsted-Lowry acid, in these reactions?
Reaction 2Which molecule acts as a Lewis acid, but NOT as a Brønsted-Lowry acid, in these reactions?
A) H2SO4
B) HNO3
C) H2NO3+
D) NO2+
Correct Answer:
Verified
Q249: Passage
Living organisms that require oxygen to respire
Q250: Passage
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
Q251: Passage
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
Q252: The first step of a nitration reaction
Q253: Passage
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
Q255: Passage
The tendency of a chemical species to
Q256: Which of the following pressure measurements is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents