Passage
Living organisms that require oxygen to respire can build up hydrogen peroxide (H2O2) as a byproduct of respiration. Because hydrogen peroxide can cause oxidative damage to cells, the amount of H2O2 in cells must be closely regulated. Although H2O2 decomposes spontaneously, a catalyst is needed for the reaction to occur at a sufficiently rapid rate suitable for biological purposes. In biological systems, an enzyme called a catalase accelerates the catalytic decomposition of H2O2 into water and oxygen (Reaction 1) .
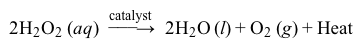 Reaction 1In a laboratory setting, several different inorganic compounds can also serve as catalysts for H2O2 decomposition, allowing researchers to compare how different catalysts affect the efficiency of the reaction. To compare Fe(NO3) 3 (a metal homogeneous catalyst) and NaI (a halogen homogeneous catalyst) , researchers measured the amount of heat evolved after placing each catalyst in a fresh solution of 3% hydrogen peroxide.To perform the measurements, the researchers placed 50 mL of 3% H2O2 in an insulated coffee cup to be used as a calorimeter, as shown in Figure 1. Before adding any catalyst, the initial, baseline temperature of the H2O2 solution was determined by recording the temperature every 30 seconds for 5 minutes. Then 10 mL of 0.10 M Fe(NO3) 3(aq) catalyst was added to the H2O2 solution. The temperature was recorded every 30 seconds for another 15 minutes. The experiment was then repeated using 10 mL of 0.50 M sodium iodide in 0.01 M NaOH as the catalyst. The results for each reaction are shown in Figure 2.
Reaction 1In a laboratory setting, several different inorganic compounds can also serve as catalysts for H2O2 decomposition, allowing researchers to compare how different catalysts affect the efficiency of the reaction. To compare Fe(NO3) 3 (a metal homogeneous catalyst) and NaI (a halogen homogeneous catalyst) , researchers measured the amount of heat evolved after placing each catalyst in a fresh solution of 3% hydrogen peroxide.To perform the measurements, the researchers placed 50 mL of 3% H2O2 in an insulated coffee cup to be used as a calorimeter, as shown in Figure 1. Before adding any catalyst, the initial, baseline temperature of the H2O2 solution was determined by recording the temperature every 30 seconds for 5 minutes. Then 10 mL of 0.10 M Fe(NO3) 3(aq) catalyst was added to the H2O2 solution. The temperature was recorded every 30 seconds for another 15 minutes. The experiment was then repeated using 10 mL of 0.50 M sodium iodide in 0.01 M NaOH as the catalyst. The results for each reaction are shown in Figure 2.
 Figure 1 Coffee cup calorimeter
Figure 1 Coffee cup calorimeter
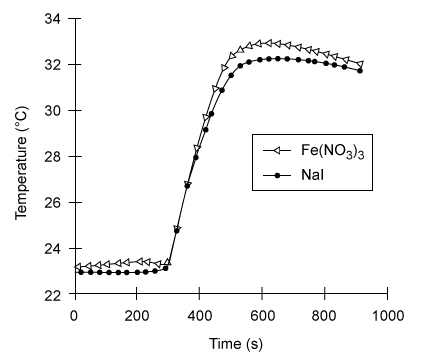 Figure 2 Temperature vs. time measurements for reactions catalyzed by Fe(NO3) 3 and NaI
Figure 2 Temperature vs. time measurements for reactions catalyzed by Fe(NO3) 3 and NaI
Adapted from: C. Marzzacco, "The effect of a change in catalyst on the enthalpy of decomposition of hydrogen peroxide." Chem13 News Magazine. ©2008 University of Waterloo.
-The experiment was repeated with 3.0 g of solid MnO2 particles added as an alternative catalyst. When MnO2 was ground to a fine powder, the temperature rose more quickly than when the particles were not ground. The most likely reason for this is that with heterogeneous catalysts:
A) the rate increases when the catalyst is oxidized.
B) the rate increases as the catalyst surface area is increased.
C) the rate decreases due to competing side reactions.
D) the rate decreases when the catalyst is insoluble.
Correct Answer:
Verified
Q254: The overall reaction for the generation of
Q255: Passage
The tendency of a chemical species to
Q256: Which of the following pressure measurements is
Q257: The equilibrium Q258: Passage Q260: Passage Q261: Passage Q262: Passage Q263: Assume that helium behaves as an ideal Q264: Atoms of a given element have a![]()
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
The tendency of a chemical species to
Sulfur is the 10th most abundant element
Sulfur is the 10th most abundant element
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents