Passage
The tendency of a chemical species to undergo reduction during an electrochemical reaction is indicated by the standard potential E° for the reaction. As represented generally by Reaction 1, E° measures the potential Z at which element X with oxidation number N accepts N electrons and is reduced to its elemental state.X(N) + Ne− → X(0) , E° = Z voltsReaction 1In a galvanic cell, E° > 0 indicates a spontaneous reaction. Therefore, chemical species with higher positive E° values are reduced more easily than those with lower E° values.Using E° as a basis of comparison, the relative thermodynamic stabilities of different chemical species involving the same element at different oxidation states can be presented graphically using a Frost diagram. For a series of compounds containing element X, a Frost diagram plots the value of NE° for each compound against the corresponding oxidation number N of element X within the species. Frost diagrams for several species containing manganese or chlorine are shown in Figure 1.
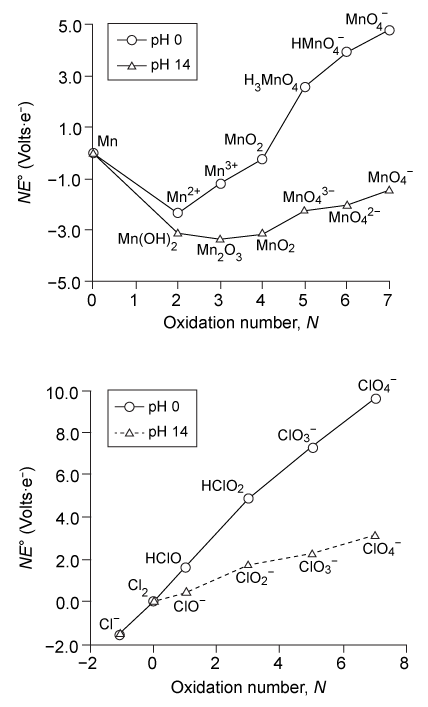 Figure 1 Frost diagrams for manganese and chlorineOn a Frost diagram, NE° is proportional to the standard Gibbs free energy ΔG° according to the relationshipΔG° = −FNE° = −nFE°Equation 1where n is the number of moles of electrons transferred during the electrochemical process and F is the Faraday constant. Free energy considerations also show that a species is prone to disproportionation if its position on the Frost diagram lies above a line connecting the points of two adjacent species, as seen for species B in Figure 2.
Figure 1 Frost diagrams for manganese and chlorineOn a Frost diagram, NE° is proportional to the standard Gibbs free energy ΔG° according to the relationshipΔG° = −FNE° = −nFE°Equation 1where n is the number of moles of electrons transferred during the electrochemical process and F is the Faraday constant. Free energy considerations also show that a species is prone to disproportionation if its position on the Frost diagram lies above a line connecting the points of two adjacent species, as seen for species B in Figure 2.
 Figure 2 General Frost diagram for an element forming species A, B, C, and D.The slope of a line segment joining two species on a Frost diagram is equal to the standard reduction potential for the couple. A greater slope indicates a higher corresponding reduction potential. As a result, in Figure 2, the reduction potential for B to A is lower than that for D to C.
Figure 2 General Frost diagram for an element forming species A, B, C, and D.The slope of a line segment joining two species on a Frost diagram is equal to the standard reduction potential for the couple. A greater slope indicates a higher corresponding reduction potential. As a result, in Figure 2, the reduction potential for B to A is lower than that for D to C.
-Formal charge and oxidation state are two different ways of accounting for electrons between atoms. Based on the Lewis structure of the ClO− anion, which of the following pairs of values corresponds to the formal charge of the chlorine atom and the oxidation state of the oxygen atom, respectively?
A) +1, −1
B) 0, −1
C) 0, −2
D) −1, −2
Correct Answer:
Verified
Q255: Passage
The tendency of a chemical species to
Q256: Which of the following pressure measurements is
Q257: The equilibrium Q258: Passage Q259: Passage Q261: Passage Q262: Passage Q263: Assume that helium behaves as an ideal Q264: Atoms of a given element have a Q265: The emission line spectra of hydrogen, lithium,![]()
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
Living organisms that require oxygen to respire
Sulfur is the 10th most abundant element
Sulfur is the 10th most abundant element
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents