The emission line spectra of hydrogen, lithium, sulfur, and selenium for the visible region of the spectrum are shown below. 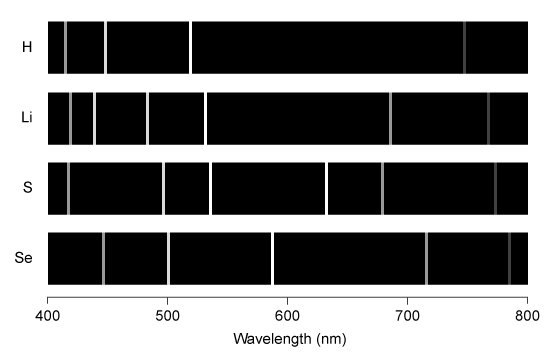 If a sample contains more than one of these elements, which of the following spectra would correspond to a sample containing both S and Se?
If a sample contains more than one of these elements, which of the following spectra would correspond to a sample containing both S and Se?
A) 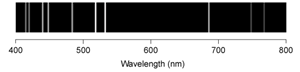
B) 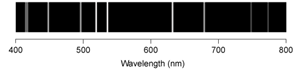
C) 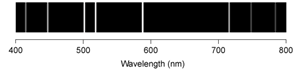
D) 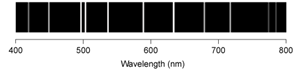
Correct Answer:
Verified
Q260: Passage
The tendency of a chemical species to
Q261: Passage
Sulfur is the 10th most abundant element
Q262: Passage
Sulfur is the 10th most abundant element
Q263: Assume that helium behaves as an ideal
Q264: Atoms of a given element have a
Q266: Passage
Students conducted an experiment to determine the
Q267: Passage
Sulfur is the 10th most abundant element
Q268: The effective nuclear charge experienced by the
Q269: Litmus paper will turn blue when immersed
Q270: Passage
Students conducted an experiment to determine the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents