Passage
Students conducted an experiment to determine the changes in enthalpy ΔH°, entropy ΔS°, and Gibbs free energy ΔG° resulting from the dissociation of acetic acid (CH3COOH) in water. The students first determined the acid dissociation constant Ka for the reaction by measuring the pH of a 1 M acetic acid solution at various temperatures over a narrow range of less than 10 °C.To perform the pH measurements, the acetic acid solution was initially cooled to 0 °C using an ice bath and then warmed with gentle heating to room temperature with continuous stirring. The pH was recorded at intervals of 1 °C. From the pH data, the value of Ka at each temperature T was calculated. The experimental results over the selected temperature range are shown in Table 1.Table 1 Student Data for the pH and Ka of Acetic Acid with Increasing Temperature
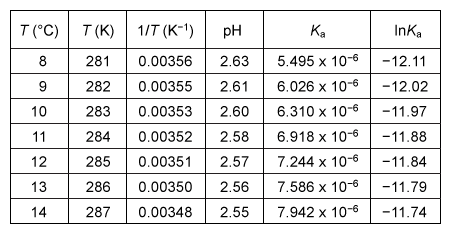 To evaluate ΔH° and ΔS°, the students constructed a van 't Hoff plot (Figure 1) , which is a graph of the natural log of the dissociation constant (ln Ka) vs. the reciprocal absolute temperature (1/T) .
To evaluate ΔH° and ΔS°, the students constructed a van 't Hoff plot (Figure 1) , which is a graph of the natural log of the dissociation constant (ln Ka) vs. the reciprocal absolute temperature (1/T) .
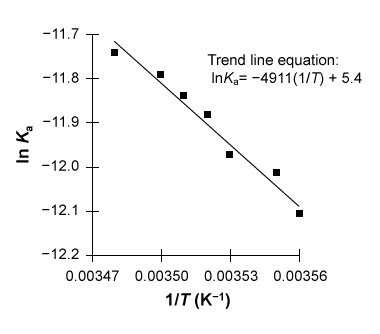 Figure 1 Linear van 't Hoff plot relating the dissociation constant Ka of acetic acid to temperature TThe relationship between Ka, T, ΔH°, and ΔS° in the van 't Hoff plot was then described using a linear fit of the data following the form of Equation 1, in which R is the gas constant (8.3 J∙mol−1∙K−1) .
Figure 1 Linear van 't Hoff plot relating the dissociation constant Ka of acetic acid to temperature TThe relationship between Ka, T, ΔH°, and ΔS° in the van 't Hoff plot was then described using a linear fit of the data following the form of Equation 1, in which R is the gas constant (8.3 J∙mol−1∙K−1) .
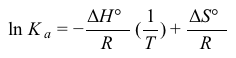 Adapted from: C. Rezsnyak "Determination of Thermodynamic Values (S°, H°, and G°) from the Dissociation of a Weak Acid" World Journal of Chemical Education. ©2017 Science and Education Publishing.
Adapted from: C. Rezsnyak "Determination of Thermodynamic Values (S°, H°, and G°) from the Dissociation of a Weak Acid" World Journal of Chemical Education. ©2017 Science and Education Publishing.
-Would changes in the van 't Hoff plot be observed if the reaction rate were increased by adding a catalyst during the experiment?
A) No, because the initial concentration of acetic acid does not change
B) No, because ΔG°, ΔH°, and ΔS° are independent of the reaction rate
C) Yes, because the reaction rate and ΔG° depend on temperature
D) Yes, because spontaneity depends on the reaction rate
Correct Answer:
Verified
Q274: Passage
Sulfur is the 10th most abundant element
Q275: A sample of potassium-42 was measured to
Q276: Passage
Students conducted an experiment to determine the
Q277: Which of the following reactions requires the
Q278: Passage
Sulfur is the 10th most abundant element
Q280: Passage
Students conducted an experiment to determine the
Q281: Passage
Oxytocin is a naturally occurring peptide hormone
Q282: The product of pressure P and volume
Q283: In chemical bond formation, covalent bonds are
Q284: Which statements correctly describe electrolysis? During electrolysis:an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents