Passage
Sulfur is the 10th most abundant element on Earth and is an essential element in biological systems. Sulfur can form a variety of chemical compounds and will react with nearly every element except Au, Pt, Ir, Te, and the noble gases. A list of several common inorganic compounds of sulfur is given in Table 1.Table 1 Selected Common Inorganic Sulfur Compounds
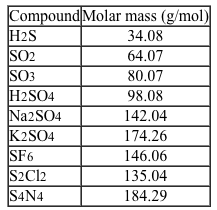 Reduction of elemental sulfur by bacteria during anaerobic digestion produces the toxic, foul-smelling gas H2S. Oxidation of elemental sulfur via combustion or volcanic processes yields gases of SO2 and SO3. When dissolved in water, both SO2 and SO3 produce acidic solutions, and SO3 reacts with water to form the acid H2SO4. Neutralization of H2SO4 can provide a variety of salts, including Na2SO4.Elemental sulfur can also react with halogen gases to form sulfur halides such as the industrially important insulator gas SF6 and the synthetic reagent S2Cl2. S2Cl2 facilitates the formation of other sulfur compounds. For example, reacting S2Cl2 with NH3 produces the sulfur pnictide S4N4.
Reduction of elemental sulfur by bacteria during anaerobic digestion produces the toxic, foul-smelling gas H2S. Oxidation of elemental sulfur via combustion or volcanic processes yields gases of SO2 and SO3. When dissolved in water, both SO2 and SO3 produce acidic solutions, and SO3 reacts with water to form the acid H2SO4. Neutralization of H2SO4 can provide a variety of salts, including Na2SO4.Elemental sulfur can also react with halogen gases to form sulfur halides such as the industrially important insulator gas SF6 and the synthetic reagent S2Cl2. S2Cl2 facilitates the formation of other sulfur compounds. For example, reacting S2Cl2 with NH3 produces the sulfur pnictide S4N4.
-Which of the compounds shown below depicts a valid Lewis structure for sulfur dioxide? (Note: Assume that the bonding of each atom involves only s and p orbitals and follows the octet rule.) 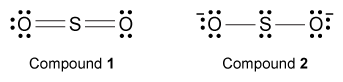
A) Compound 1 only
B) Compound 2 only
C) Both compounds
D) Neither compound
Correct Answer:
Verified
Q273: Passage
Students conducted an experiment to determine the
Q274: Passage
Sulfur is the 10th most abundant element
Q275: A sample of potassium-42 was measured to
Q276: Passage
Students conducted an experiment to determine the
Q277: Which of the following reactions requires the
Q279: Passage
Students conducted an experiment to determine the
Q280: Passage
Students conducted an experiment to determine the
Q281: Passage
Oxytocin is a naturally occurring peptide hormone
Q282: The product of pressure P and volume
Q283: In chemical bond formation, covalent bonds are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents