The product of pressure P and volume V of an ideal gas is directly proportional to the product of the moles n and absolute temperature T of the gas, as shown below. 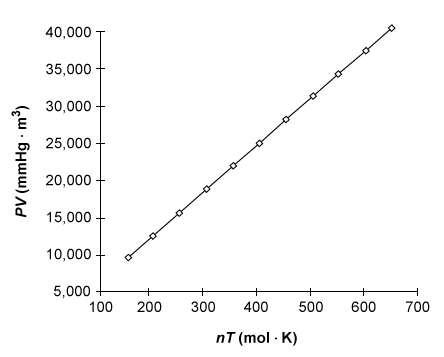 What is the value of the gas constant R derived from the graph of the data?
What is the value of the gas constant R derived from the graph of the data?
A) 0.0160 mol∙K/mmHg∙m3
B) 0.0821 L∙atm/mol∙K
C) 8.31 J/mol∙K
D) 62.5 mmHg∙m3/mol∙K
Correct Answer:
Verified
Q277: Which of the following reactions requires the
Q278: Passage
Sulfur is the 10th most abundant element
Q279: Passage
Students conducted an experiment to determine the
Q280: Passage
Students conducted an experiment to determine the
Q281: Passage
Oxytocin is a naturally occurring peptide hormone
Q283: In chemical bond formation, covalent bonds are
Q284: Which statements correctly describe electrolysis? During electrolysis:an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents