A test used by geologists to determine if the carbonate ion is present in rock can be performed by dripping a hydrochloric acid solution on the sample and looking for the bubbling of carbon dioxide. How many moles of carbon dioxide are produced by the reaction of 0.75 moles of limestone, CaCO3? 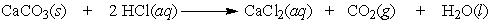
A) 2.35 moles
B) 2 moles
C) 1 mole
D) 0.75 moles
Correct Answer:
Verified
Q38: According to the following equation, what mass
Q39: Methanol burns in air according to the
Q40: Balance the equation below and determine the
Q41: How many grams of oxygen (O2) are
Q42: In the following reaction, how many grams
Q44: The percent yield of a reaction is
Q45: If 2.00 grams of potassium are allowed
Q46: A chemist runs a reaction to prepare
Q47: If there is a "limiting reactant" in
Q48: Sodium stearate is a soap that is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents