If 2.00 grams of potassium are allowed to react with 2.00 grams of bromine by the following equation, which reactant is the limiting reactant? 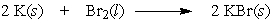
A) K
B) Br2
C) cannot be determined
Correct Answer:
Verified
Q40: Balance the equation below and determine the
Q41: How many grams of oxygen (O2) are
Q42: In the following reaction, how many grams
Q43: A test used by geologists to determine
Q44: The percent yield of a reaction is
Q46: A chemist runs a reaction to prepare
Q47: If there is a "limiting reactant" in
Q48: Sodium stearate is a soap that is
Q49: What mass of potassium chloride, a salt
Q50: While this isn't a real reaction, we
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents