Sodium stearate is a soap that is produced from stearic acid, one of the fatty acids, by the reaction: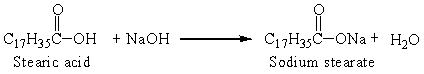 The reaction that was set up with 0.25 g stearic acid and an excess of sodium hydroxide produced 0.25 grams soap. The percent yield was
The reaction that was set up with 0.25 g stearic acid and an excess of sodium hydroxide produced 0.25 grams soap. The percent yield was
A) 100%.
B) 93%.
C) 25%.
D) 1%.
Correct Answer:
Verified
Q43: A test used by geologists to determine
Q44: The percent yield of a reaction is
Q45: If 2.00 grams of potassium are allowed
Q46: A chemist runs a reaction to prepare
Q47: If there is a "limiting reactant" in
Q49: What mass of potassium chloride, a salt
Q50: While this isn't a real reaction, we
Q51: Two methanol molecules can react in the
Q52: Ethene, C2H4, burns in air according to
Q53: Consider the burning of methane gas (CH4):
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents