A phase diagram, like the one below, for an aqueous solution shows a shift in the solid/liquid and liquid/gas phase transitions but there is not a shift in the sublimation line between solids and gases. Explain what causes the shift in the other transitions and why this does not change a shift in the solid/gas transition.Hint: Think about the difference in vapor pressure between a solution and a pure substance. 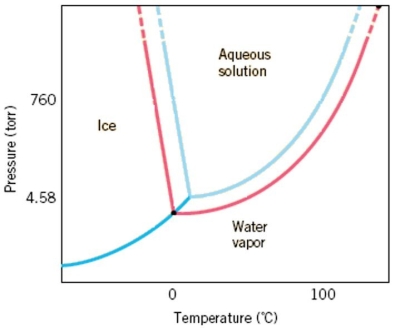
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q123: Pure benzene, C6H6, has a molar mass
Q124: A solution is made by mixing acetone
Q125: Pure cyclohexane, C6H12, has a molar mass
Q126: Pure cyclohexane, C6H12, has a molar mass
Q127: Pure glacial acetic acid, HC2H3O2, has a
Q128: Pure benzene, C6H6, has a molar mass
Q129: An aqueous solution of fructose, C6H12O6, has
Q130: A solution is made by dissolving 48.07
Q131: How many liters of ethylene glycol antifreeze
Q132: A chemist isolates a new compound with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents