In a gas mixture containing oxygen, neon, and argon, the mole fractions are: 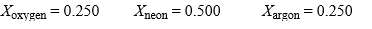 If this mixture behaves as an ideal gas, what is the density of this mixture, in g/liter, at 37.0 °C and 765 torr? If someone gave a sample of the mix described above to a student and had them determine the molar mass of the "gas"without telling the student it was a mixture, what value should the student report?
If this mixture behaves as an ideal gas, what is the density of this mixture, in g/liter, at 37.0 °C and 765 torr? If someone gave a sample of the mix described above to a student and had them determine the molar mass of the "gas"without telling the student it was a mixture, what value should the student report?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q151: People who live on the second floor
Q152: A general chemistry student needs to heat
Q153: Why do a person's ears "pop"when changing
Q154: Five moles of oxygen gas are heated
Q155: Calculate the volume of hydrogen gas at
Q157: A piece of zinc is allowed to
Q158: Individuals are often advised to put out
Q159: During a chemical demonstration, a filled balloon
Q160: Water vapor is a polar gas molecule.
Q161: Explain how real gases differ from ideal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents