The thermochemical equation for the reaction between dinitrogen monoxide and oxygen to produce nitrogen dioxide is shown below. Write the thermochemical equation for the reaction when 1.00 mole of nitrogen dioxide is formed. 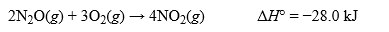
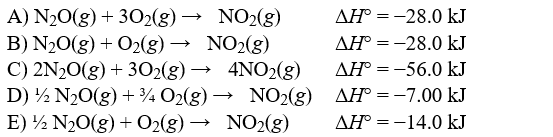
Correct Answer:
Verified
Q53: When 0.250 moles of LiCl are
Q54: What would be the "standard state"for acetic
Q55: What would be the "standard state"for hydrogen
Q56: When nitrogen gas reacts with hydrogen
Q57: When aluminum metal reacts with iron(III)oxide
Q59: The combustion of butane, C4H10, is given
Q60: Propane is often used to heat homes.
Q61: For the reaction below: Q62: When nitrogen gas reacts with hydrogen Q63: Consider the following thermochemical equation: ![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents