Multiple Choice
When nitrogen gas reacts with hydrogen gas to form ammonia, 92.38 kJ of heat is given off for each mole of nitrogen gas consumed, under constant pressure and standard conditions. What is the value of H° for the reverse of the reaction shown? 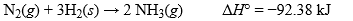
A) +34.5 kJ
B) -46.19 kJ
C) +59.2 kJ
D) -59.2 kJ
E) +92.38 kJ
Correct Answer:
Verified
Related Questions
Q57: When aluminum metal reacts with iron(III)oxide
Q58: The thermochemical equation for the reaction between
Q59: The combustion of butane, C4H10, is given
Q60: Propane is often used to heat homes.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents
