Determine the standard enthalpy change, H°, for the reaction, CCl4(l) + 4HCl(g) CH4(g) + 4Cl2(g) , given the following thermochemical equations: 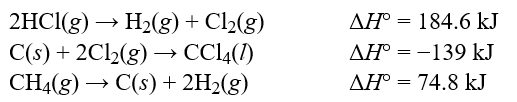 Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.
Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.
A) +55.3 kJ
B) -187 kJ
C) +101 kJ
D) -179 kJ
E) +433 kJ
Correct Answer:
Verified
Q62: When nitrogen gas reacts with hydrogen
Q63: Consider the following thermochemical equation: 
Q64: Determine the enthalpy change, ?H, for
Q65: Determine the enthalpy change,
Q66: Determine the enthalpy change, ?H, for
Q68: The thermochemical equation that is associated
Q69: The thermochemical equation that is associated
Q70: The thermochemical equation that is associated
Q71: The thermochemical equation that is associated
Q72: The thermochemical equation that is associated
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents