Determine the enthalpy change, ?H, for the reaction, CS2(l) + 3O2(g) CO2(g) + 2SO2(g) , given the following thermochemical equations: 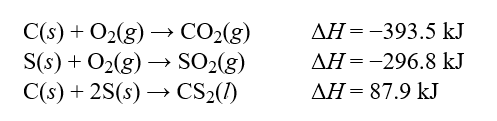 Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.
Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.
A) +778.2 kJ
B) -602.4 kJ
C) -1075 kJ
D) -778.2 kJ
E) +602.4 kJ
Correct Answer:
Verified
Q61: For the reaction below: Q62: When nitrogen gas reacts with hydrogen Q63: Consider the following thermochemical equation: Q64: Determine the enthalpy change, ?H, for Q65: Determine the enthalpy change, Q67: Determine the standard enthalpy change, Q68: The thermochemical equation that is associated Q69: The thermochemical equation that is associated Q70: The thermochemical equation that is associated Q71: The thermochemical equation that is associated![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents