The thermochemical equation for the reaction between hydrazine, N2H4, and dinitrogen tetroxide is given as: 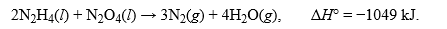 .What is the corresponding thermochemical equation for this reaction when 6 mol of nitrogen are formed?
.What is the corresponding thermochemical equation for this reaction when 6 mol of nitrogen are formed?
Correct Answer:
Verified
Q99: For a chemical reaction in which the
Q100: At constant pressure, the difference between the
Q101: When 0.250 moles of KCl are added
Q102: The thermochemical equation for the reaction between
Q103: The thermochemical equation for the reaction between
Q105: The thermochemical equation for the reaction of
Q106: The thermochemical equation for the reaction of
Q107: For the reaction of graphite with oxygen
Q108: The combustion of butane, C4H10, is given
Q109: The thermochemical equation for the reaction of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents