The combustion of butane, C4H10, is given as: 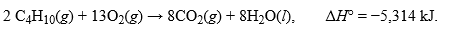 .How many grams of butane must be reacted by this reaction to release 10,525 kJ of heat?Hint: Use your coefficients from the equation and convert to moles as an intermediary when performing your calculations.
.How many grams of butane must be reacted by this reaction to release 10,525 kJ of heat?Hint: Use your coefficients from the equation and convert to moles as an intermediary when performing your calculations.
Correct Answer:
Verified
Q103: The thermochemical equation for the reaction between
Q104: The thermochemical equation for the reaction between
Q105: The thermochemical equation for the reaction of
Q106: The thermochemical equation for the reaction of
Q107: For the reaction of graphite with oxygen
Q109: The thermochemical equation for the reaction of
Q110: For the reaction, N2(g)+ 3 H2(g)→ 2
Q111: For the reaction, 3 N2(g)+ H2(g)→ 2
Q112: Given the thermochemical equation, 2 M2O5(s)→ 4
Q113: Given the thermochemical equation, 3 M(s)+ 3
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents