The thermochemical equation for the reaction of sulfur dioxide, SO2, with oxygen is given as: 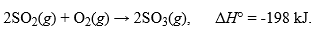 .What is the change in enthalpy when 5 moles of SO2 react with 2 moles of oxygen by this reaction?Hint: Use the coefficients in the equation and the mole ratio shown during your calculation.
.What is the change in enthalpy when 5 moles of SO2 react with 2 moles of oxygen by this reaction?Hint: Use the coefficients in the equation and the mole ratio shown during your calculation.
Correct Answer:
Verified
Q101: When 0.250 moles of KCl are added
Q102: The thermochemical equation for the reaction between
Q103: The thermochemical equation for the reaction between
Q104: The thermochemical equation for the reaction between
Q105: The thermochemical equation for the reaction of
Q107: For the reaction of graphite with oxygen
Q108: The combustion of butane, C4H10, is given
Q109: The thermochemical equation for the reaction of
Q110: For the reaction, N2(g)+ 3 H2(g)→ 2
Q111: For the reaction, 3 N2(g)+ H2(g)→ 2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents