Using the equation shown, 7A + 5B → 3C + 4D, and the standard enthalpies of formation, ΔHf °: 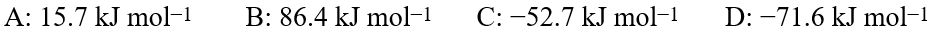 calculate ΔH°reaction in kJ for the hypothetical reaction above.
calculate ΔH°reaction in kJ for the hypothetical reaction above.
Correct Answer:
Verified
Q111: For the reaction, 3 N2(g)+ H2(g)→ 2
Q112: Given the thermochemical equation, 2 M2O5(s)→ 4
Q113: Given the thermochemical equation, 3 M(s)+ 3
Q114: Use these reactions and standard enthalpies, ΔH°
2
Q115: Use these reactions and standard enthalpies, ΔH°
2
Q117: Using the standard enthalpies of formation, ΔH°f:
Q118: Complete combustion of hydrocarbons or compounds with
Q119: Complete combustion of hydrocarbons or compounds with
Q120: Complete combustion of hydrocarbons or compounds with
Q121: Using the standard enthalpies of formation, ΔH°f:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents