Multiple Choice
Describe the relationship between the rate constants kf and kr for the following one step reaction at equilibrium.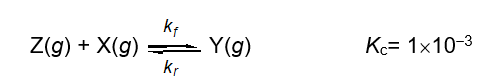
A) kf = kr
B) kf < kr
C) kf > kr
D) kf = kr = 0
E) none of these
Correct Answer:
Verified
Related Questions
Q1: The following reaction was carried out:
Ni(CO)4
Q3: For the reaction:
CO(g) + Cl2(g) 

