Multiple Choice
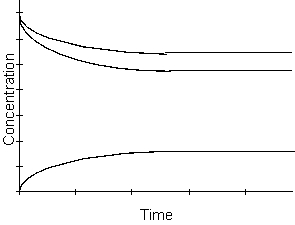
-What happens to the rate of the reaction as time passes in the equilibrium region of the graph above?
A) the rate increases
B) the rate decreases
C) the rate remains constant
D) cannot be determined from the graph
Correct Answer:
Verified
Related Questions
Q1: The following reaction was carried out:
Ni(CO)4
Q2: Describe the relationship between the rate constants
Q3: For the reaction:
CO(g) + Cl2(g) 

