Multiple Choice
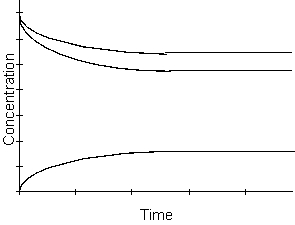
-From the graph above, which of the following is true when equilibrium has been reached?
A) The concentrations of the reactants are larger than the concentration of the products.
B) The concentrations of the products are larger than the concentration of the reactants.
C) The concentrations of the reactants and products are equal.
D) This cannot be determined from the graph.
Correct Answer:
Verified
Related Questions
Q2: Describe the relationship between the rate constants
Q3: For the reaction:
CO(g) + Cl2(g) 

