Use the following standard enthalpies of atom combination to
Hac° H2(g) = -435.30 kJ/molrxn Hac F2(g) = -157.98 kJ/molrxn
Hac° N2(g) = -945.41 kJ/molrxn Hac° C(s) = -716.68 kJ/molrxn
Hac° O2(g) = -498.34 kJ/molrxn Hac° NH3(g) = -1171.8 kJ/molrxn
Hac° H2O(g) = -926.3 kJ/molrxn Hac° HF(g) = -567.7 kJ/molrxn
-What is the bond energy of N 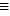 N?
N?
A) 237 kJ/mol
B) 158 kJ/mol
C) 473 kJ/mol
D) 946 kJ/mol
E) 1892 kJ/mol
Correct Answer:
Verified
Q59: (Note that some of these
Q60: The reaction below shows two isomers of
Q61: Retailers purchase gasoline by weight so they
Q62: Which of the following is not true
Q63: Write a balanced equation for the
Q64: Use the following standard enthalpies of
Q66: Use the following standard enthalpies of
Q67: Use the following standard enthalpies of
Q68: Use the following standard enthalpies of
Q69: Consider the following data for heats of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents