On the energy diagram below,which arrow(s) represent the activation energy for the forward and reverse reactions? 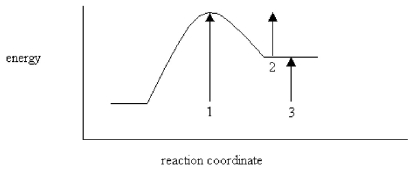
A) Arrow 1 is the activation energy for both the forward and reverse reactions.
B) Arrow 1 is the activation energy for the forward reaction and arrow 2 is the activation energy for the reverse reaction.
C) Arrow 1 is the activation energy for the forward reaction and arrow 3 is the activation energy for the reverse reaction.
D) Arrow 3 is the activation energy for the forward reaction and arrow 2 is the activation energy for the reverse reaction.
Correct Answer:
Verified
Q14: Which statement does not apply to transition
Q15: Which amino acid is least likely to
Q16: In the following chemical reaction which species
Q17: On the energy diagram below,which point represents
Q18: Replacement of the amino acid _ at
Q20: An enzyme stabilizes the transition state that
Q21: The following pH dependence was found for
Q22: When the concentration of a substrate inside
Q23: Superoxide dismutase enzyme catalysis is faster than
Q24: A reaction that occurs with every collision
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents