A container is divided into two chambers that are separated by a valve.The left chamber contains one mole of a monatomic ideal gas.The right chamber is evacuated.At some instant,the valve is opened and the gas rushes freely into the right chamber.Which one of the following statements concerning this process is true? 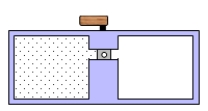
A) Work is done by the gas.
B) The temperature of the gas decreases.
C) The change in the entropy of the gas is zero.
D) The walls of the containing vessel must get colder.
E) The change in the internal energy of the gas is zero.
Correct Answer:
Verified
Q1: If one complete cycle of a reversible
Q2: A thermally isolated sample of an ideal
Q4: A system containing an ideal gas at
Q5: Neon is a monatomic gas with a
Q6: 5.00 kg of liquid water is heated
Q7: Which one of the following pressure-volume graphs
Q8: When the gas enclosed beneath the piston
Q9: Enclosed beneath the moveable piston in the
Q10: Which one of the following situations is
Q11: An ideal gas absorbs 750 J of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents